

|
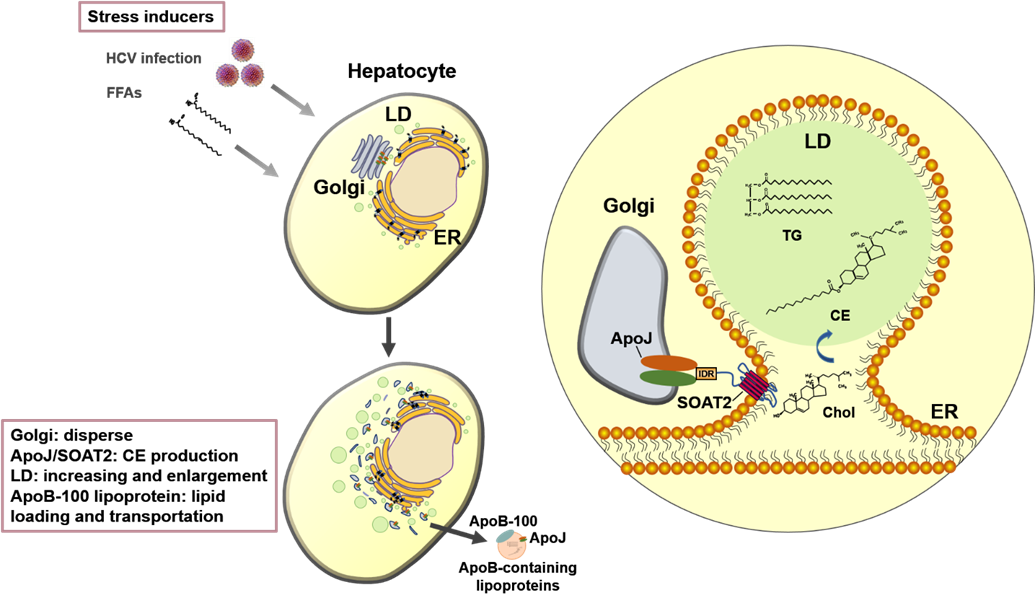
Leading to the common road of virus- and obesity-induced fatty liver Increasing virus infection, obesity and a combination of both diseases threaten human health globally, prompting innovative biomedical research of our team to investigate the interplay of infectious pathogens and host metabolism in the development of either diagnostics or therapeutics. Apolipoprotein J (ApoJ) was identified on the common pathways of hepatic lipid accumulation caused by both virus (hepatitis C virus) and obesity (fatty acids) inducers. |
|||||||
| _______________________________________________________________________________________________ | |||||||
|
Our team in the Center of Infectious Disease and Signaling Research studied the characterization of lipoprotein components, and successfully identified the molecular roles of Apolipoprotein J (ApoJ), which participates in dual parts to facilitate hepatitis C virus (HCV) infection and to establish aberrant hepatic lipid accumulation. If the hepatic lipid deposit exceeds 5%, the liver becomes steatosis (fatty liver), which can progress to steatohepatitis, then cirrhosis, hepatocellular carcinoma, and simultaneously increase the risks of high blood pressure, diabetes and chronic kidney disease. Non-alcoholic fatty liver disease (NAFLD) challenges healthcare systems in the world due to the increased adoption of westernized lifestyle, especially in aging, over-weight or obese populations, however, currently proven treatment is still limited. Lipoproteins consist of various components including lipids and apolipoproteins, whose primary function serve as plasma transport vesicles. Different from the extracellular errands of apolipoproteins, our team characterized the intracellular functions of ApoJ at lipid droplet-endoplasmic reticulum-Golgi membrane contact site where it serves as chaperon for infectious HCV particle production. Exposures of HCV or free fatty acids could concomitantly disrupt Golgi structure, induce closer ApoJ contact with sterol O-acyltransferase 2, increase cholesteryl-ester biogenesis and hepatic lipid droplet accumulation. In addition, serum ApoJ correlated positively with cholesterol and low-density lipoprotein levels in normal glycaemic HCV patients, NAFLD patients and in mice with steatosis. The therapeutic peptides were designed to target ApoJ chaperone activity for blockade of hepatic lipid overload, creating a new strategy for the treatment of virus- and obesity-induced fatty liver.
|
|||||||
|
Fig. 1. ApoJ is a glucose-upregulated molecular chaperon, at lipid droplet-endoplasmic reticulum-Golgi membrane contact site stabilizes core and NS5A to promote infectious HCV virion production |
|||||||
|
Figure 2. Knockdown of ApoJ prevents (A) FFA- and (B) HCV-induced intracellular lipid overloads in hepatocytes |
|||||||
|
Figure 3. Correlations of circulating ApoJ, serum TC, and LDL in CHC patients, in NAFLD patients, and in the mouse with steatosis. |
|||||||
|
Figure 4. Treatment with (A) ApoJ antagonist peptide could (B) reduce hepatic lipid overloads in mice with steatosis. |
|||||||
| _______________________________________________________________________________________________ | |||||||
|
|||||||